The process of separating the components of a boiling mixture.
At the beginning of boiling, the gas phase in equilibrium with the liquid mixture contains primarily the most volatile product. This vapour is enriched in the most volatile product by successive condensations in a fractionating column and then it is completely condensed and recovered in an external container.
Atmospheric
pressure distillation
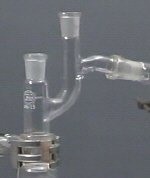
Distillation head
Fractionnal distillation
Reduced pressure distillation
Steam distillation
Atmospheric
pressure distillation
In this procedure the liquid boils at atmospheric pressure.

The part of the distillation apparatus which makes it possible to vary the flow rate of the liquid being distilled.
It is placed between the column and the condenser and commonly contains a means of measuring the temperature of the liquid being distilled, e.g. a thermometer pocket.
.

A distillation process of distillation where several stages of evaporation and condensation occur during which the phase vapour becomes enriched in the most volatile product and the liquid phase in the least volatile products. The process allows liquids with similar boiling points to be separated.

Fractionating columns




When the products to be purified decompose at high temperature, the distillation is carried out under reduced pressure so as to lower their boiling temperatures.
The reduced pressure in a distillation apparatus is achieved by connecting the apparatus to a pump, e.g. a water pump or a vacuum pump.
Boiling is controlled by having a capillary bleed into the distillation flask using air or nitrogen depending on whether the substance to be distilled is prone to oxidation..

This is a process used to purify substances which decompose above 100°C, have a significant vapour pressure pi below 100°C, and are insoluble in water. The liquid mixture made up of an aqueous phase and the impure product is boiled at atmospheric pressure, i.e.
Psample + Pwater = 1 atm.
As the liquid boils and the vapour phase is separated, condensed and collected in an external container. If the impure substance is a liquid then the two phases which are distilled can be separated to recover the purified product.
