Entropy
Denoted by S.
The entropy (S) is function of state defined by the relationship:
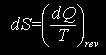
in which dS is the change in the entropy , dQ is a reversible heat absorption by the system, and T is the temperature at which that absorption takes place.
For a transformation taking place from an initial state i to a final state f, whatever the path followed (S being related to state)
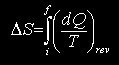
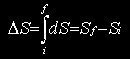
for a reversible path.
For other transformations, it is necessary to devise a reversible path leading from the same initial state i to the same final state f.
Entropy of reaction
When a system undergoes a chemical conversion; the entropy of reaction corresponds to the difference in entropy between the products of the reaction and the entropy of the reagents necessary for their production.